Medical Isotope Applications
Radioactive isotopes are invaluable for the diagnosis and treatment of diseases like cancer. When radioactive atoms are attached to carefully chosen carrier molecules, small doses of radiation can be directed to body parts or cancerous tumors which allow physicians to image the operations of organs or deliver fatal doses of radiation to malignant cells. While these medical radioisotopes are currently produced using nuclear reactors or accelerators, many promising new candidates are unable to reach the clinic due to difficulty in their production or preparation for use in patients. The development of IsoDAR’s state-of-the-art cyclotron technology can have far-reaching applications to medical isotope production, which would allow for increased domestic manufacturing capabilities making radiopharmaceuticals more accessible to those that need them.
Medical Isotope Production
The technology behind IsoDAR's cyclotron can be adjusted to produce radiopharmaceutical isotopes. IsoDAR works by accelerating H2+, having a charge-to-mass ratio of 1:2, to 60 MeV per nucleon. With minimal reconfiguring, such a cyclotron can be adjusted to accelerate virtually any species having the same charge-to-mass ratio (such as deuterons or C6+) to an energy between 5 and 60 MeV per nucleon. This technology introduces an unprecedented flexibility in isotope production. When one of these machines is combined with one or multiple targets (made possible by beam splitting, where small pieces of the beam can be "shaved off" by electron stripping and diverted to different targets), a wide range of radioactive isotopes can be produced. Two isotopes especially exciting to the IsoDAR collaboration, Ge-68 and Ac-225, can have significant medical applications.
Isotopes of Interest
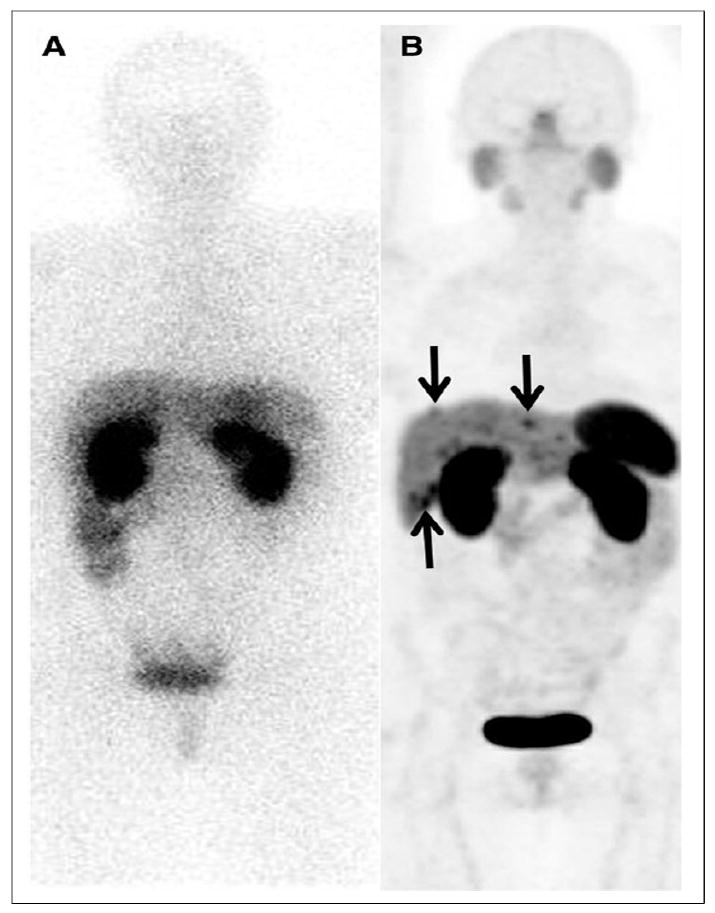
A PET (positron emission tomography) scan works by injecting a radioactive tracer into the body, which emits positrons that collide with electrons, producing gamma rays of characteristic energy. These gamma rays are detected by the PET scanner to create detailed images of metabolic activity within the body, an exceptional diagnostic tool. A commonly used isotope for PET imaging is gallium-68, which has a half-life of just 68 minutes. To produce Ga-68, one can produce Ge-68, which has a (relatively long) half-life of 270 days and decays by positron emission to Ga-68. Ge-68 is said to be a “generator” of Ga-68. Crucially, Ge-68’s long shelf-life ensures that the parent isotope is prevented from decaying during transportation to a treatment facility; however, its long half-life means that producing the daughter Ga-68 isotope is costly and time-consuming. Ge-68 can be produced by irradiating a natural gallium target with protons. At IsoDAR’s nominal acceleration capability of 10 mA of 60 MeV protons, one can produce approximately 50 Ci of Ge-68 in a just a week of runtime, substantially reducing the cost of this kind of diagnostic imaging.
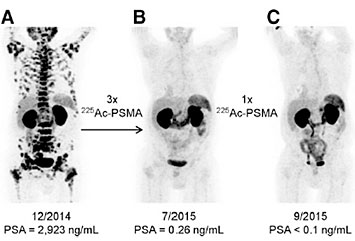
Another isotope of interest is actinium-225, which has been shown to be highly effective in treating certain cancers. Ac-225 has a half-life of 9.9 days and includes a four alpha particle decay chain which provides powerful and localized radiation damage needed to kill the malignant cells of a tumor. Ac-225 can be produced from the beta decay of Ra-225 (half-life 14 days), a byproduct of the alpha decay of Th-229 (half-life 8,000 years). If a radium salt is paired with a beryllium target for a deuteron beam, the beam's incidence on the beryllium can cause the deuteron to break up, into a proton and a neutron. These fast neutrons can then react with the radium via a (n,2n) reaction to produce Ac-225.